1. Where Do COVID-19 Vaccines Come from?
On August 26, 2021, the Ministry of Health, Labour and Welfare announced the suspension of the use of some lots of Moderna's COVID-19 vaccine due to the presence of contaminants in vials. According to media reports, potentially contaminated lots of the vaccine were manufactured by Rovi of Spain and were imported into Japan (Nihon Keizai Shimbun, August 26, 2021). This article examines the situation of imports of COVID-19 vaccines and clarifies how much of the imports into Japan come from Spain.
2. Vaccine Imports Viewed from Trade Statistics
The situation of vaccine imports can be identified on a monthly basis from trade statistics announced by the Ministry of Finance. Import source countries can be identified from item-by-item, country-by-country tables included in the trade statistics. As of August 30, 2021, data for the period until July 2021 were publicly available.
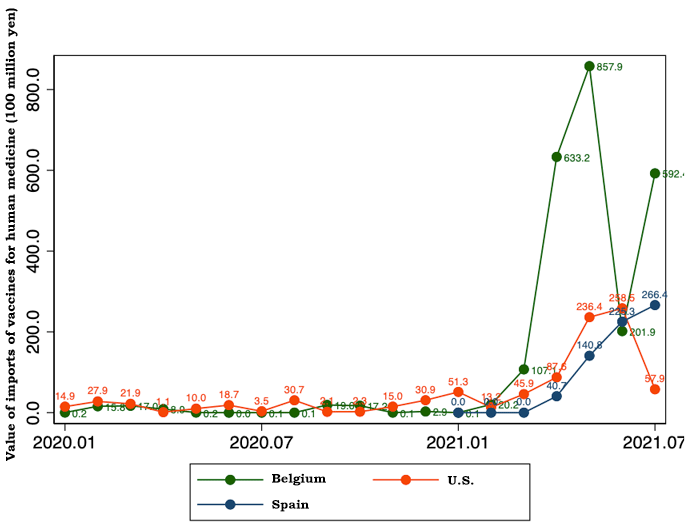
In the trade statistics, COVID-19 vaccines are considered to be classified into the "vaccines for human medicine" category under HS Code (Harmonized System Code for import-export items) 300220000. This category includes various vaccines other than COVID-19 vaccines. Despite this statistical constraint, Figure 1 shows that the value of vaccine trade in 2021 increased steeply compared with 2020. The monthly value of vaccine imports ranged from around 2 billion yen to 6 billion yen during 2020 but surpassed 20 billion yen in March 2021 and later: 20.9 billion yen in March, 77 billion yen in April, 126.3 billion yen in May, 78.7 billion yen in June, and 94.3 billion yen in July. Most of the increase in the value of vaccines imports may be considered to be attributable to imports of COVID-19 vaccines. While the value of vaccine imports increased steadily from March to May, it declined in June and July. As will be mentioned later, the decline is due to steep drops in imports from Belgium in June and from the United States in July.
(January 2020 to July 2021)
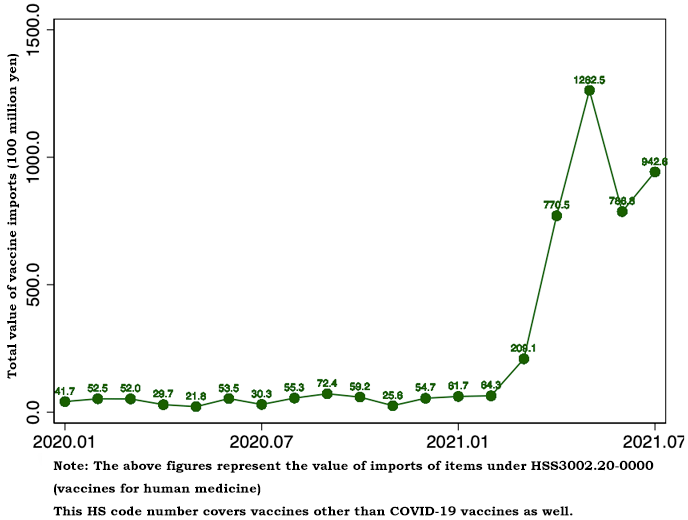
In terms of "volume," vaccine imports in 2021 did not necessarily increase steeply compared with 2020. The apparent lack of any significant change presumably reflects the fact that COVID-19 vaccines are negligibly small in volume while being expensive.
3. Vaccine Imports from Spain
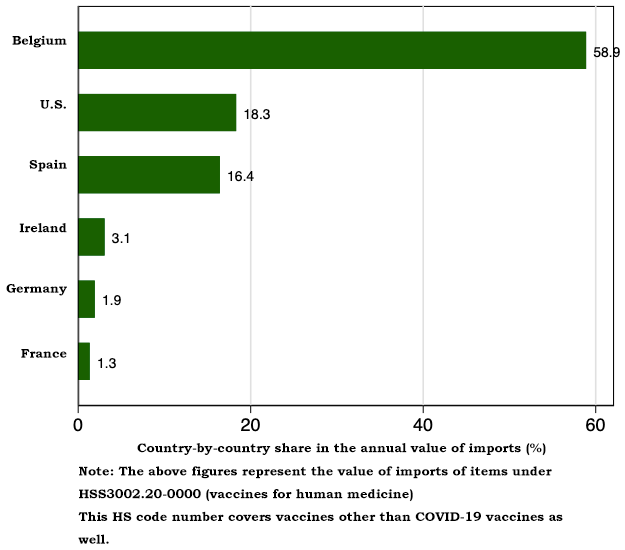
According to the trade statistics, between January and July 2021, Japanese imports of vaccines for human medicine came mainly from the following six countries: Belgium (58.9%), the United States (18.3%), Spain (16.4%), Ireland (3.1%), Germany (1.9%) and France (1.3%). Other import source countries' shares are miniscule. Spain is the third largest import source country. In terms of value, 241.3 billion yen's worth of vaccine imports came from Belgium, 75.1 billion yen's worth from the United States, 67.3 billion yen's worth from Spain, 12.6 billion yen's worth from Ireland, 7.9 billion yen's worth from Germany, and 5.5 billion yen's worth from France. The value of Japanese vaccines imports since the beginning of 2021 was already huge sums as of July.
(January-July 2021)
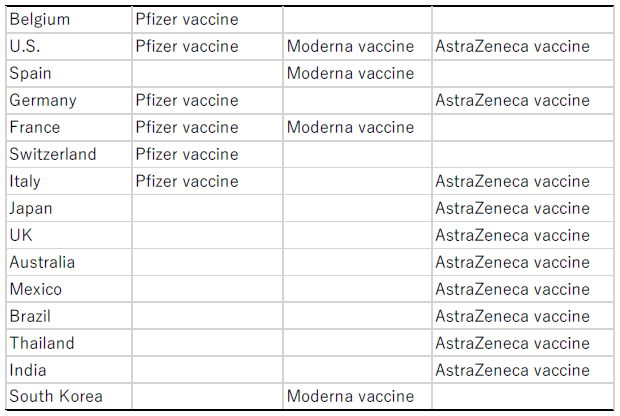
The Moderna vaccine is presumed to account for the whole of imports from Spain. That is because, according to Bown and Bollyky (2021), as of the end of June 2021, the final process of the manufacturing process of the Pfizer vaccine was implemented only in the United States, Belgium, Germany, France, Switzerland, and Italy, as shown in Table 1. Meanwhile, the whole of 30 million doses' worth of the AstraZeneca vaccine concentrates that had been imported into Japan before March 2021 came from the United States, with the remaining 90 million doses' worth to be manufactured in Japan, according to a media report (Asahi Shimbun, April 1, 2021).
On the other hand, the final process of the manufacturing of the Moderna vaccine is implemented in the United States, Spain, France and South Korea. In light of those pieces of information, it is presumed that vaccine imports from Belgium are entirely of the Pfizer vaccine, imports from the United States include both the Pfizer and/or Moderna and AstraZeneca vaccines, and imports from Spain are entirely of the Moderna vaccine.
(As of the end of June 2021)
From Belgium, the United States, and Spain, which are the main vaccine import sources for Japan as explained above, how many did Japan import by July? According to media reports, the price of one dose of the AstraZeneca vaccine is around 450 yen (Asahi Shimbun, March 16, 2021), while the price of one dose of the Pfizer or Moderna vaccine is around 2,000-2,800 yen (Nihon Keizai Shimbun, August 2021, TV Asahi news, November 11, 2020, etc.)
Table 2 shows the estimated import volumes in the period until July calculated on the vaccine price assumptions based on the media reports and on the assumption that COVID-19 vaccines account for the whole of the import value of vaccines for human medicine. Under those assumptions, the Japanese government may be presumed to have procured more than 160 million doses' worth of vaccines by July through imports from Belgium, Spain and the United States alone. Given that two doses are necessary for each person in the case of the Pfizer or Moderna vaccine, this volume is sufficient to fully inoculate around 80 million people. In addition, 90 million doses' worth of the AstraZeneca vaccine are planned to be manufactured in Japan, according to a media report (Asahi Shimbun, April 1, 2021).
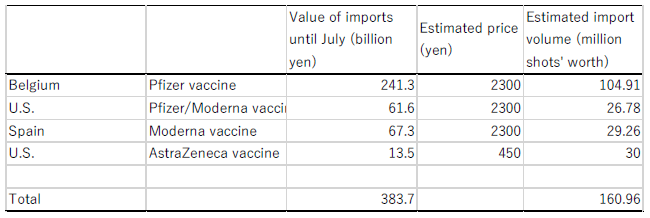
Assumption 1: COVID-19 vaccines account for the whole of the value of imports of vaccines for human medicine.
Assumption 2: The price of the AstraZeneca vaccine per shot is 450 yen.
Assumption 3: The price of the Pfizer or Moderna vaccine per shot is 2,300 yen.
Figure 3 shows that while vaccine imports from the United States declined steeply in July, imports from Spain consistently increased from April to July 2021. Imports from Spain may have made up for the drop in vaccine supply from the United States. If imports from Spain are completely suspended, workplace vaccination programs using the Moderna vaccine are expected to be considerably affected. Imports from Belgium, which is presumed to be a supply source of the Pfizer vaccine, decreased steeply in June but rebounded in July.
(January-July 2020)
4. Global Vaccine Exports
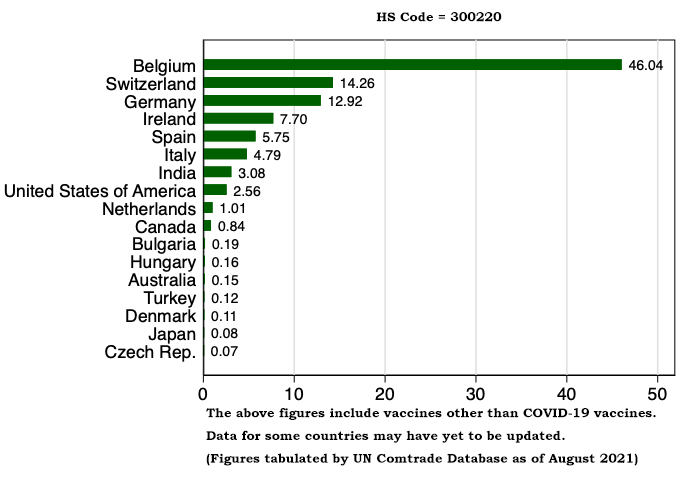
According to UN Comtrade trade statistics (monthly), published by the United Nations based on figures reported by individual countries, Spain is presumed to have a significant share of global exports of vaccines for human medicine. Of the global total of exports of vaccines for human medicine in August 2021 as published by the United Nations, which was 11.0 billion dollars, exports from Spain accounted for 0.63 billion dollars. Spain's share of global exports of vaccines for human medicine in the period from January to May 2021 was 5.8% based on the published export value as shown in Figure 4, although this is an incomplete, provisional figure, given the delays in reports from some countries to the United Nations.
(January-May 2021)
5. Supply Chain for Vaccine Production and Future Challenges
The manufacturing processes of COVID-19 vaccines are dispersed across national borders. According to Bown and Bollyky (2021), in the case of the Moderna vaccine, as of June 2021, vaccine concentrates manufactured in the United States, Switzerland, and France were refined into a drug substance in the United States, Switzerland, Spain, and the Netherlands, while the fill and finish process was implemented in the United States, Spain, France or South Korea.
Japan is not participating in the international division of work in the manufacturing of the Moderna vaccine. Japan lacks sufficient pharmaceutical production capacity. If calculated based on data from the Statistics of Production by Pharmaceutical Industry (Ministry of Health, Labour and Welfare), Japan's domestic vaccine production ratio in 2019 was 61.2%. If vaccines manufactured mostly from imported substances are excluded from the calculation, the domestic vaccine production ratio was 48.1%. Given the growing demand for vaccines, it is important to increase vaccine production capacity, but it may not be easy to do that immediately. In order to ensure safety, Japan should consider how it can participate in vaccine production, at least in the fill and finish process as the first step.
The original text in Japanese was posted on August 30, 2021.


