The COVID-19 pandemic has reminded us of the importance of the administration of the government’s healthcare policy. Promptly developing and introducing effective vaccines and therapeutic drugs is the most important challenge for enhancing the people’s well-being. Bearing that in mind, the Institute for New Era Strategy (INES), of which the authors are members, examined the current state of and the challenges for the drug market and the drug pricing system and conducted a study on a new pricing system.
The growth of the drug market in Japan is expected to slow rapidly, and there is even the possibility that the market may shrink depending on the course of events. According to a forecast by IQVIA Japan, the estimated annual growth rate of the drug market in FY2020-2026 ranges from minus 0.8% to plus 0.2%. Overseas, the estimated growth rate in FY2019-2025 is in the 4-7% range for each of China and South Korea, the 2-5% range for the United States and the 3-6% range for Germany.
The main reason for the low growth rate in Japan relative to the rates elsewhere is that drug prices in the country have been lowered to the degree that they have offset the organic growth of the market. Given the aging of society with a shrinking population of children and a rapidly declining working-age population in Japan, the price reductions in recent years may be reasonable to some degree. However, the declining attractiveness of the Japanese market in the eyes of drug companies, the stagnation of innovation, and the slow pace of new drug introduction are causes of serious concern.
Here, we regard the drug pricing system as a link through which industrial policy and fiscal policy (insurance finance) in the area of social security are intertwined. The government needs to maintain the universal insurance system and ensure market growth even amid the shrinking of the population and, at the same time, keep the system fiscally sustainable through drug cost optimization. We are presenting a three-pronged reform proposal as a key to realizing an ideal drug pricing system.
◆◆◆
The first thing to do would be to introduce a new drug price calculation method that appropriately reflects the innovative value of new drugs when publishing regulated drug prices. The objective is to resolve problems involved in the cost-based calculation method, which has been adopted by Japan alone among major countries. Under that method, drug prices are determined based on figures obtained by adding various expenses and the profit margin to the manufacturing cost. Now that the open innovation approach has become widespread as a means of drug development, it is difficult to identify the manufacturing cost. It is even more difficult to ensure that the enormous cost of drug discovery is reflected in prices.
As the first step, drug companies would develop a highly valid calculation method and data that places emphasis on the evaluation of innovation while looking at overseas cases for reference. Based on the prepared data, they would hold negotiations with the regulatory authority while bearing the burden of proof in presenting their argument for their pricing.
Second, it is necessary to abolish, in principle, the requirement that the price of a drug be lowered when the drug’s annual sales exceed the level anticipated initially by drug companies (the requirement that the price be revised when the drug’s indications have changed should be maintained). Although that rule is effective as a way of maintaining the sustainability of social insurance finance, prices of effective drugs under patent protection would automatically have to be lowered merely because the drugs sell more than initially anticipated. As a result, innovative companies may be deprived of legitimate profit opportunities. From the drug companies’ point of view, this could be a factor that discourages the introduction of advanced drugs.
Some people may be worried that this rule change would benefit drug companies and would cause the overall medication cost to rise out of control. Therefore, it is important to create a new pricing framework under which the upper limit on the growth of overall drug spending is predetermined in accordance with the macroeconomic growth rate and corporate resource allocations are made under that limit with respect to the evaluation of the value of new drugs and investment recovery.
Under our proposed framework, an “overall drug cost adjustment mechanism” would be introduced. Specifically, when market demand for a new drug has proved to be higher than expected, lowering the drug’s price would not take precedence as is the case under the present pricing system. Rather, a price adjustment would be made broadly, with minimal price reduction applied to many drugs, mainly for “long-term listed drugs” that have lost patent protection, and generic drugs.
Under this framework, drug cost would be allowed to increase in line with the growth rate of gross domestic product (GDP). The abovementioned cost adjustment mechanism would be triggered in order to keep the growth in overall drug cost within the threshold level only if the growth in cost overshoots the GDP growth due to special factors, such as the arrival of a ground-breaking drug.
The threshold rate of growth in overall drug cost would be set in line with medium-to long-term government forecasts for the GDP growth rate (average rate), such as estimates by the Cabinet Office. For the period until around fiscal year 2030, the government currently forecasts an annual growth rate of at least around 1% in nominal GDP, a figure that is higher than the expected growth rate of the drug market that was mentioned earlier. As a result, the drug market in Japan would become more attractive.
If nominal GDP grows 1%, tax revenue and social insurance premium revenue also grow around 1%. If overall drug cost increases in line with the medium- to long-term estimates of the GDP growth rate, no additional burden would be imposed on the people and the sustainability of healthcare insurance finance would be maintained. Another benefit of the proposed framework is that inflation is factored in, which means that increases in raw materials prices can be reflected in drug price revisions, unlike under the current drug pricing system.
More specifically, the overall limit on drug cost would be set in accordance with the threshold drug cost growth rate (Z%) agreed in advance based on forecasts. Drugs would be classified into three categories: (i) the innovative drug category, (ii) the mature drug category, and (iii) the basic drug category. At the time of regular drug price revisions, revisions would be made with respect to all three categories, as has been the case until now. A revised drug price would be calculated through the following formula: The revised price = the market price + (the pre-revision designated price) x (the adjustment multiple).
Let us assume that a new ground-breaking drug has been introduced, resulting in a much higher growth rate of the drug cost than the predetermined threshold growth rate (Z%). In this case, the price adjustment mechanism would be applied not to the innovative drugs category but to the mature drug category in order to keep the overall drug cost within the limit. In other words, the mechanism would be applied only to drugs with a relatively low level of innovation, while the innovative and basic drug categories, which contribute to the growth of the market, would be exempted from application. Prices will be lowered based on the “macroeconomic slide” adjustment rate, which is intended to keep the cost within the overall cost limit.
This means that a new price adjustment method that keeps the cost growth below the threshold growth rate would be applied to mature drugs, using the calculation formula of “the revised price = the market price + (the pre-revision designated price) x (the macroeconomic slide adjustment rate).” When the growth in overall drug cost is below the threshold growth rate, the macroeconomic-slide adjustment would not be made in order to allow the cost to increase in line with the threshold growth rate.
◆◆◆
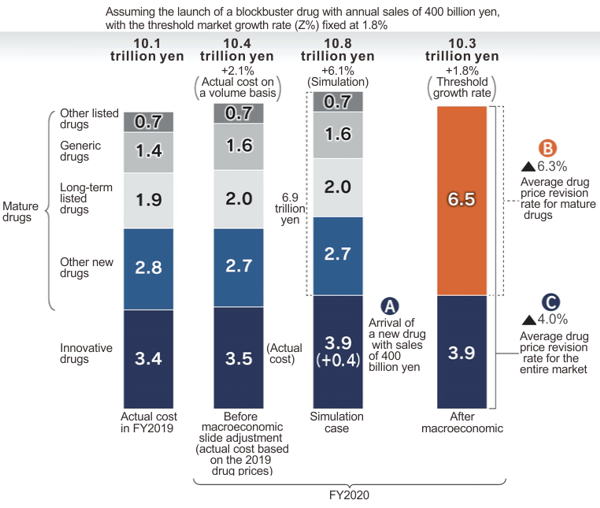
The figure below shows the results of analysis of a simulation assuming the application of the overall drug cost adjustment mechanism to the FY2020 drug cost, which was conducted by INES using data prepared by IQVIA.
[Click to enlarge]
According to the trial calculation, even if sales of the innovative drug category increased by 400 billion yen because of additional sales of a new blockbuster drug, the average price revision rate for the entire drug market would be minus 4%, while the average price revision rate for the mature drug category would be minus 6.3%. These figures are lower than the actual revision rates under the current regular annual price revision.
The greatest reason for this is that the overall drug cost growth adjustment mechanism has the effect of eliminating fiscal uncertainty over future insurance finance while increasing overall drug cost in line with the potential medium- to long-term GDP growth rate.
Our proposal described above should serve as a basis for future discussions. We hope that vigorous discussions will be held, including on our proposal, while the basic principles of the reform of the drug pricing system indicated by Prime Minister Kishida Fumio—emphasizing innovation and ensuring the sustainability of the universal insurance system—are respected.
* Translated by RIETI.
June 28, 2022 Nihon Keizai Shimbun



