The new coronavirus poses a major threat to the human race at the present moment, since it exhibits both high infectivity and a high mortality rate, while no proven therapeutic agents or vaccines exist. However, we have observed significant efforts to repurpose existing drugs and drug candidates for treatments, because the new coronavirus has similar mechanisms of proliferation and can creates "cytokine storms" as in other diseases for which effective treatments are available. Thus, the newly generated demand for new treatments may allow repurposed drugs to provide quick therapeutic responses. This short column examines how such opportunities arise and what may hinder full exploitation of such opportunities. We focus on BCG, Avigan (generic name: favipiravir) and Actemra (generic name: tocilizumab), which have been widely regarded as promising candidates for medicines treating COVID-19. The last two were discovered in Japan. Japan also retained the early BCG strain which is very close to the original BCG, which is more potent than other strains, in particular than the Denmark strain, which was widely used in Europe (Miyasaka, 2020).
Enhancing the natural immune system with BCG or its derivative
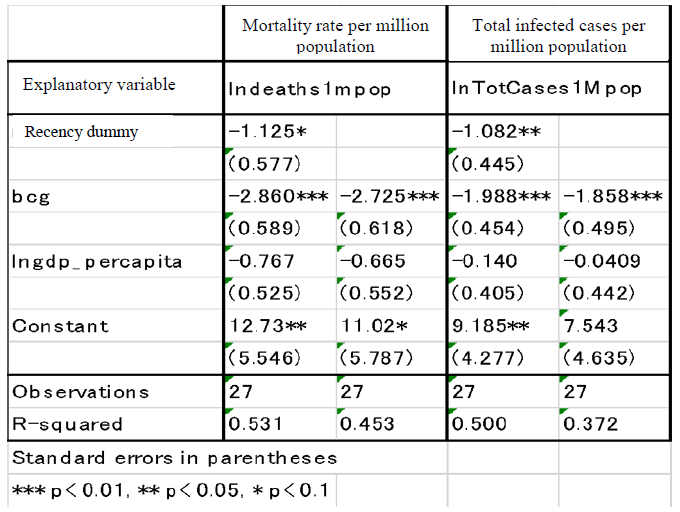
BCG, a vaccine that has proved highly effective in eradicating tuberculosis and is widely used for its prevention, is known for its ability to boost the natural immune system (Hirano, 2020). Thus, it can reduce both infection and death rates from the novel coronavirus. The safety of the vaccine is also well established. Clear and substantial differences between countries are observed in the mortality rates from new coronavirus, depending on whether the county has universally implemented BCG vaccination. Table 1 illustrates the differences in mortality and infection rates among the 27 countries with the highest number of people infected by the novel coronavirus, based on the following criteria: 1) the recency dummy for the onset of infection (i.e., whether the difference between the date of the first confirmed infection case and that in China is less than 30 days); 2) the universal BCG vaccination status (bcg = 1 if BCG vaccinations has been universally administered; 0 if otherwise); and 3) the gross domestic product per person (GDP per capita, PPP: Purchasing Power Parity). In countries where BCG vaccinations have been administered universally, both mortality and infection rates are much lower at high statistical significance, controlling the other two explanatory variables. For example, the coefficient of the logarithm of the mortality rate of -2.9 indicates that the mortality rate is approximately one-sixteenth less, while that of the infection rate of -2 indicates that the infection rate is one-seventh less. The countries with recent onsets of infection exhibit lower mortality and infection rates, as expected, while the coefficients in the GDP per capita are negative, which is not significant.
(https://en.wikipedia.org/wiki/COVID-19_pandemic_by_country_and_territory).
The status of BCG universal vaccination in each country was prepared by the author based on Zwerling, 2011. GDP per capita for each country is sourced from the data in "The World Bank Atlas of Sustainable Development Goals."
The correlation between mortality rates and BCG vaccination status is not necessarily causal, for the mortality rate depends on other factors such as the speed and the intensity of implementing social distancing policy and level of healthcare resources in each country. Only clinical trials can establish the causality (Note 1) According to the WHO database (COVID-19 Studies from the World Health Organization Database), 7 randomized, large scale (1,000 patients enrollments) clinical trials are under way as of May 2020, centered around universities in Australia, the Netherlands, the United States, Brazil and others, and 5 of them are placebo-controlled. If the BCG vaccine can be validated to be effective, its injection may become a valuable option especially in those countries with no universal BCG vaccination, since the development of specific vaccines against the novel coronavirus will require time. In Japan, the vaccination rate of BCG vaccine is as high as 98%, but its potency declines with age, so a secondary BCG vaccination for the elderly, who are most vulnerable to the new coronavirus, may become an option.
Utilizing existing antiviral drugs
The second therapeutic strategy is to repurpose therapeutic agents for other infectious diseases, in order to prevent either entry or replication of new coronavirus within the host's cells. Avigan (favipiravir), discovered by a Japanese pharmaceutical company (FUJIFILM Toyama Chemical), is a promising antiviral drug inhibiting the proliferation of the new coronavirus, which was suggested by the results of a comparative clinical trial conducted in China (Shiraki 2020b). Guy et al. (2020) assesses Avigan (favipiravir) as one of the best justified drugs for repurposing to treat COVID-19 patients, based on the existing evidence.
Avigan (favipiravir) was originally developed as an influenza drug. It was discovered in 1998 and it took many years before it was finally approved in 2014. It is characterized by its effectiveness against lethal influenza infections and its excellent property that it does not contribute to the development of resistant viruses, due to its direct inhibition of RNA duplications in the cells. However, there are already many existing drugs in the anti-influenza medication market, and the sales of these drugs are highly volatile, depending on the level of the spread of a given flu. Due to these circumstances, Toyama Chemical suspended its development for an extended period of time. However, clinical trials for Avigan (favipiravir) were resumed partly because of the discovery by a U.S. university of its efficacy against avian influenza disease (Shūkan Gendai, 2014). After being acquired by FUJIFILM and obtaining funding to continue clinical trials, with support from the U.S. Department of Defense, Toyama Chemical finally managed to obtain approval for Avigan (favipiravir). However, its approval was subject to the following two conditions. It must not be prescribed for pregnant patients and its use must be limited to the cases of diseases for which other anti-influenza viral drugs are ineffective, due to the fact that animal studies suggest damage to early embryos (Note 2). The Japanese government has secured a stockpile for emergency use.
Since it was approved only as an anti-influenza drug in Japan, Avigan requires a separate clinical trial for use against COVID-19. FUJIFILM Toyama Chemical began clinical trials and are conducting phase 3 trials in Japan, and phase 2 in the US. The comparison with the clinical trials of Remdesivir which was discovered by Gilead science and is another drug that inhibits the RNA duplication of the new coronavirus, suggest that the scale and speed of the clinical trials for Avigan (favipiravir) were limited. Remdesivir was already approved in early May in the US (later in Japan) on the basis of emergency use authorization, based on the results of international clinical trials covering more than 800 patients. On the other hand, the phase 3 clinical trial for Avigan is still ongoing, aiming at covering a sample size of 96 patients for Japan, as of June 2020. The rapid reduction of the number of the patients from the first wave of COVID-19 has made it difficult to recruit patients, so that the phase 3 is now expected to continue into July 2020.
We can identify three potential sources of differences between the trials. The first is the difficulty of implementing placebo-controlled clinical trials in Japan for patients who are seriously ill. While Avigan (favipiravir) were prescribed to more than 2,000 patients in the context of clinical research (drugs provided by the government) in Japan, no effective placebo existed by design, so that the clinical research could only confirm its safety. The treatment of patients was given much higher priority than the scientific investigation of its efficacy. The second is the patent position. While the product patent for Avigan (favipiravir) has expired, that of Remdesivir remains effective. The effective patent would presumably allow the firm to globally coordinate the clinical trials and to obtain long-term returns for the launches, once the effectiveness is well recognized. Third, the US government was more directly involved in the clinical trials of Remdesivir through the National Institute of Allergy and Infectious Diseases becoming the primary sponsor of the placebo-controlled, international, randomized and blind clinical trials for Remdesivir.
Utilizing therapeutic drugs for autoimmune diseases
The third therapeutic strategy is to use existing treatments for autoimmune diseases to control the excessive release of inflammatory cytokines in seriously ill patients. The new coronavirus can cause acute respiratory distress syndrome (ARDS), which can lead to death. An important cause of such illness is a cytokine storm (cytokine release syndrome), in which virally infected cells stimulate immune-related cells to release inflammatory cytokines (e.g., IL-6 and TNF-α), further activating immune cells. This in turn promotes further cytokine release, causing a destructive cycle of uncontrolled inflammation. This excessive release of inflammatory cytokines results in serious damage to the lungs and other organs (Hirano, 2020). Successful suppression of the cytokine storm can be expected to result in a substantial reduction in mortality.
Cytokine overproduction is not a newly-discovered syndrome. It is known to cause rheumatoid arthritis and other autoimmune diseases, and cytokine inhibiting drugs that are highly effective in the treatment of rheumatoid arthritis have already been developed. Among such drugs, Actemra (tocilizumab), which was developed by Osaka University and Chugai Pharmaceutical through a long-term collaborative partnership (Note 3), is a promising drug in combating a cytokine storm caused by the novel coronaviruses. Actemra (tocilizumab) is the first inhibitor against the IL-6 signal pathway.
As for the status of clinical trials for Actemra (tocilizumab), Chugai's parent company, Roche, is conducting a large scale international and placebo-controlled phase-III clinical trial (330 patients in 11 countries), and its subsidiary Genentech is also conducting a complementary large scale phase-III clinical trial. Furthermore, in the United States, clinical trials with universities, foundations, university hospitals, etc. as sponsors are also progressing concurrently. In Japan, Chugai is preparing to conduct a domestic phase III study in hospitalized patients with severe new coronavirus pneumonia as of May 2020.
Conclusions
While the new coronavirus poses a major threat to the human race at the present moment, there are a number of existing drugs, the repurposing of which could significantly reduce the threat. In other words, the pharmaceutical innovations of the past have benefits that were unanticipated at the time of their discovery.
To make the unexpected benefits a reality, rapid implementation of clinical trials are crucial. The experience in Japan suggests that it is not necessarily easy, when the treatment of the patients is given much higher priority than the scientific investigation of its efficacy, which made it difficult to implement placebo-controlled clinical trials. Furthermore, the rapid reduction in the number of patients from the first wave of COVID-19 in Japan has made it difficult to recruit new patients. International collaborations in clinical trials would be very important not only for the rapid diffusion of the drugs with successful drug repurposing, but also to exploit the economies of scale and scope of clinical trials, as demonstrated in the cases of Remdesivir and Actemra (tocilizumab).
The rapid repurposing of existing drugs suggests that demand is a major constraint for pharmaceutical innovations. This is the case because clinical trials are essential investments for pharmaceutical innovation and they are very costly. At the same time, repurposing of the existing drugs has become possible only because of the existence of a stock of drugs with diverse mechanism of actions. Both Avigan (favipiravir) and Actemra (tocilizumab) were developed through extended periods of research and development, with collaboration with industry and academia. It is important to reaffirm the value of drug discovery efforts underlined by basic research that investigates fundamental mechanisms of action.
* I thank Dr. OHSUGI Yoshiyuki (Ohsugi BioPharma Consulting) for providing valuable suggestions for the preparation of this research paper.


