Unique international law
The Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from their Utilization (hereinafter referred to as the "Nagoya Protocol") was adopted in 2010 in Nagoya for the purpose of complementing the 1992 Convention on Biological Diversity, and it was agreed that the protocol should be in force and operational by 2015. Chances of meeting this deadline seemed to have disappeared, but recent developments in Europe changed the tide. The Council of the European Union (EU) adopted the EU Regulation on Compliance Measures for User from the Nagoya Protocol (Note 1) (hereinafter referred to as the "EU Regulation"), and EU member states are now beginning to take steps for the ratification of the protocol. As such, the coming months will witness a sudden increase in the number of countries having ratified the protocol, increasing the likelihood of its coming into force before the end of this year (Note 2). Japan is also aiming to ratify the protocol at an early date, and a study is currently underway on domestic implementation measures (Note 3).
The Nagoya Protocol is a unique international law whereby signatory countries would commit themselves to monitoring the utilization of genetic resources by users within their respective jurisdictions for compliance with relevant laws and regulations of provider countries, and, in the case of non-compliance, taking appropriate remedial measures (e.g., imposition of penalties) in accordance with their own domestic laws and regulations. Once it is brought into effect, users of genetic resources will be checked by the authorities of their own countries as well as those of provider countries. Users will be required to exercise a considerable degree of diligence even in the case of utilizing genetic resources obtained through third parties, not to mention the case where they collect such resources on their own. Therefore, users may be forced to bear an unreasonably heavy burden depending on the nature of domestic compliance measures implemented by their home countries.
The legislative challenge on the part of user countries is how to fulfill their international commitment while at the same time minimizing the burden on users. This article aims to review the compliance measures adopted by the EU Regulation and sort out the key points that will likely provide useful insights to Japan in considering its domestic compliance measures (Note 4).
Terminologies crucial to understanding the EU Regulation
"Utilization of genetic resources": Article 2 (c) of the Nagoya Protocol defines this term as meaning "to conduct research and development on the genetic and/or biochemical composition of genetic resources, including through the application of biotechnology." Based on this definition, which is also applied in the EU Regulation, for instance, importing and selling imported fruits would not be considered an act of utilizing genetic resources, whereas developing drugs by conducting a biochemical analysis of fruits would be considered as such. Meanwhile, use of such drugs by a third party as material for other products would not constitute an act of utilizing genetic resources (Note 5).
With respect to "traditional knowledge associated with genetic resources" and the "utilization" thereof, the EU Regulation does not provide specific definitions (due to the absence of international consensus), leaving the matter to contractual arrangements concluded between parties concerned (Article 3, paragraph 7 of the EU Regulation) (Note 6).
"Access and Benefit-Sharing Clearing House": This refers to an international portal site to be established in accordance with Article 14 of the Nagoya Protocol for the purpose of sharing information related to access and benefit sharing (ABS). The names of competent national authorities, ABS-related legislative, administrative, and policy measures, and other relevant information will be provided on the portal site.
"Internationally-recognized certificate of compliance": Under the Nagoya Protocol, provider countries would be required to issue a written permit to users as evidence of: (1) the decision to grant prior informed consent and (2) the establishment of mutually agreed terms (MAT) on benefit sharing. Such a permit made available to the Access and Benefit-Sharing Clearing House would constitute an internationally-recognized certificate of compliance (Article 17, paragraph 2 of the Nagoya Protocol).
"Register of collections": An accumulation of genetic resources or traditional knowledge associated with such resources is called a "collection," and notifying the public of collections verified to meet the designated criteria is referred to as the "register of collections" (Article 5 of the EU Regulation). The criteria for register require a collection to demonstrate its capacity, inter alia, to: (a) apply standardized procedures for providing samples of genetic resources to users; (b) supply genetic resources only with documentation providing evidence that such resources were accessed lawfully; and (c) keep records of all samples of genetic resources supplied to users.
"Due diligence": Generally, this term refers to the degree of care that a reasonable person would exercise in taking action. In the context of the EU Regulation, it refers to users' obligation in utilizing genetic resources to ascertain that: (1) genetic resources which they utilize have been accessed in accordance with applicable laws and regulations of the provider country and (2) benefits arising from the utilization of such resources are to be shared fairly and equitably upon mutually agreed terms (Article 4, paragraph 1 of the EU Regulation).
"Recognition of best practices": Under the EU Regulation, associations of users may submit an application to the European Commission to have their ABS compliance measures—i.e., those developed and overseen by them—recognized as a "best practice" defined under the regulation (Article 8 of the EU Regulation). The European Commission would grant such recognition where it determines that the effective implementation by a user of such measures enables the user to comply with its obligations under the EU Regulation, i.e., obligations to exercise due diligence and declare to the competent authorities that it has fulfilled its obligations with a submission of evidence thereof. The Conference of the Parties to the Nagoya Protocol may also adopt best practices (Article 20 of the Nagoya Protocol). These two kinds of best practices will be listed and on an online-based register to be established by the EU.
Register of collections and the easing of the burden of due diligence
As a way to strike a balance between the two goals of fulfilling obligations to the international community and easing the burden of users, the EU Regulation calls for establishing a register system for reliable collections whereby users obtaining genetic resources from a registered collection would be considered as having exercised due diligence.
Users obtaining genetic resources through any route other than the one described above would be required to follow and demonstrate due diligence. However, even in this case, presenting an internationally-recognized certificate of compliance would be considered as constituting an effective demonstration of due diligence so as to minimize the burden of users (see the figure below). Though not included in the figure, the EU Regulation also provides that users obtaining certain plant genetic resources for food and agriculture would be deemed to have exercised due diligence (Note 7).
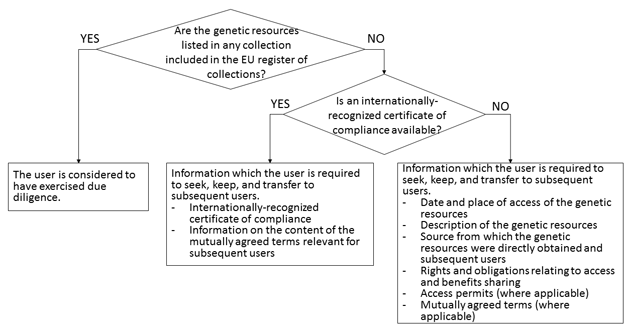
[Click to enlarge]
In short, users would bear the burden of due diligence only when they obtain genetic resources through a route other than via the EU register of collections and an internationally-recognized certificate of compliance is not available. In such a case, users would be required to seek, keep, and transfer to subsequent users the information listed in the box in the bottom right corner of the figure. And if they are unable to provide such information or uncertainties regarding the legality of access and utilization persist, they would have to discontinue utilization (Article 4, paragraph 5 of the EU Regulation).
Recognition of best practices and streamlining of the monitoring process
Under the EU Regulation, monitoring is implemented on a self-declaration basis. For instance, those users supported by research funding would be required to declare, at the time of receiving the funds, that they exercise due diligence. In addition, at the stage of final development of a product developed by utilizing genetic resources, all users would be required to declare to the competent authorities that they have exercised due diligence (Article 7 of the EU Regulation).
Of course, this is not meant to leave everything to users. The competent authorities would carry out checks on users' compliance as appropriate. However, this checking mechanism is rational and streamlined in the following two aspects.
First, the implementation of an EU-recognized best practice would be taken into account to apply a streamlined checking procedure to those users adopting such a practice (Article 9, paragraph 1 of the EU Regulation). Second, rigorous checks would be carried out where non-compliance is doubted. Specifically, checks would be conducted: (a) in accordance with a periodically reviewed plan developed using a risk-based approach (Note 8); and (b) when a competent authority is in possession of relevant information, including on the basis of substantiated concerns provided by third parties or provider countries, regarding a user's non-compliance (Article 9, paragraph 3 of the EU Regulation).
In the case of shortcomings in compliance, users would be subject to penalties under the domestic law of the EU member state to which they belong. However, it should be noted that competent authorities would not be allowed to impose such penalties in a unilateral manner. A prior notice would be issued to non-complying users in order to give them a chance to correct the situation (Article 9, paragraph 6 of the EU Regulation).
Other points of attention
First, the EU Regulation says that it "applies to genetic resources over which states exercise sovereign rights and to traditional knowledge associated with genetic resources that are accessed after the entry into force of the Nagoya Protocol for the (European) Union." The latter part of this text, which is to preclude retroactive application, is reasonable. However, "states exercise sovereign rights" in the first part should be rephrased as "states exercise sovereign rights in accordance with the Convention on Biological Diversity" so as to exclude cases where states exercise in violation of the convention. Also, it is necessary to consider explicitly defining "states" referred to therein as meaning "states that are parties to the Nagoya Protocol."
Second, in the process of drafting the EU Regulation, the term "genetic resources" was initially defined to include "derivatives." Eventually, however, the EU decided to follow the definition prescribed in Article 2 of the Nagoya Protocol, thereby excluding derivatives from the scope of genetic resources. Japan should consider and draw on this course of events in the EU as a reference in developing its own legislation to implement the protocol.
Third, the EU Regulation does not include explicit provisions for certain genetic resources for which appropriate ABS measures are deemed to be implemented through customary practices or implicit agreements within industries (e.g., medicinal plants). However, it should be provided that users of such genetic resources will be deemed to be fulfilling their compliance obligations.
Fourth, Article 4, paragraph 8 of the EU Regulation provides for specific due diligence obligations for those utilizing a pathogen as well as measures taken in the case of non-fulfillment such as the suspension of patents, and such provisions are not necessarily a crucial requisite under the Nagoya Protocol. Presumably, those provisions are a product of political compromise within the EU. Indeed, as a legal text, the provisions contain some flaws (Note 9). As such, there is no need for Japan to follow this example. Instead, it should explore a different approach (for instance, leaving the matter to the relevant arrangements provided for by the World Health Organization (WHO)).
Fifth, regarding the time limit for users' declaration of their having exercised due diligence, the EU Regulation provides that the "stage of final development of a product," at which time users will be required to make such declaration, shall be determined separately by the European Commission in implementing acts (Article 7, paragraph 6 of the EU Regulation). It should be also noted that those provisions concerning due diligence declaration would be applied after a grace period of one year from the entry into force of the Nagoya Protocol. As such, there is no need to do everything all at once and Japan should consider pursing a step-by-step approach, whereby certain provisions would be applied on a later date as appropriate based on the implementation status of the initial set of provisions.
What is expected of the government and users
It is understandable that the Japanese government (Ministry of the Environment) wants to push for early ratification as the host of the 10th meeting of the Conference of Parties (COP10) to the Convention on Biological Diversity, which adopted the Nagoya Protocol. However, the protocol is a product of political compromise and, depending on the way it is implemented, would have an enormous—or at least unpredictable—impact on biotechnology research and development in user countries. Therefore, my personal belief is that the government should take a step-by-step approach in considering domestic measures for the ratification of the protocol, starting with the minimum steps required to fulfill its commitment to the international community and taking more steps along the path based on the evaluation of the effect and impact of earlier steps.
We should also acknowledge the fact that the Japan Bioindustry Association (JBA) has been developing and promoting best practices, for instance, through the publication of Iden Shigen eno Akusesu Tebiki [Guidelines on Access to Genetic Resources] (Note 10) prior to the adoption of the Nagoya Protocol (Note 11). So, hurrying up with the legislation of domestic measures is not the only way to go. The Japanese government should be able to fulfill its obligation as the host country of COP10 by promoting rational and constructive implementation models in the world while concurrently appealing the existing voluntary and effective initiatives by users' associations in Japan such as the one by JBA.
At the same time, Japanese users must change their mindset and reform their systems. The time when genetic resources belonged to all human beings and could be utilized by anyone for free is long gone. They need to examine the relevant laws and regulations of provider countries prior to accessing genetic resources and must act in compliance with such laws and regulations, which have now become an international rule. It is hoped that Japanese users—particularly universities and public research institutions—will expedite their efforts to develop internationally acceptable best practices by using the JBA guidelines and other available materials as reference.
The original text in Japanese was posted on May 21, 2014.
June 11, 2014


