PDF version [PDF:577KB]
Importance of Innovative Drugs as Carved Out by the Novel Coronavirus
The global outbreak (pandemic) of novel coronavirus infections has once again brought recognition of the importance of the development of pharmaceuticals such as vaccines and treatment drugs. During the current crisis, had vaccines by Pfizer or Moderna not been developed at this high speed, the challenges posed by the coronavirus infections would have been much more serious.
Pharmaceuticals have an important role to play in protecting the health and lives of people from danger in various situations. However, there is also a lack of transparency in the pharmaceutical manufacturing field due to the frequent changes to the drug pricing system. In recent years, the sophistication of medical care has led to the development of innovative drugs. Even though they fulfill the healthcare needs of people, they are often accompanied by high prices, and there are concerns about the increase in drug costs and their fiscal impact.
In particular, in Japan, where a declining population, a declining birthrate and an ageing society is progressing, government debt is accumulating and in view of fiscal consolidation, new fiscal restrictive measures against the growth in social security related expenditures, such as medical care, have been implemented since the 2010s.
One such symbolic measure is thought to be the “Basic Polices” (the official title is “Basic Policies for Economic and Fiscal Management and Structural Reform”). In Basic Policies 2018, the fiscal surplus of the Primary Balance (PB) for fiscal 2025, which combines the national and regional surpluses, has been set as a fiscal consolidation target. To achieve this target, a policy has been determined to restrain the real increase in social security-related expenditures to the level equivalent to an increase due to the ageing society, and efforts to restrain growth have been made at the annual drafting of the budget.
What is most frequently used to constrain this growth in social security expenditures is the “revision of drug prices”. For example, in the initial budget (general account for Japan) for fiscal 2022, in order to keep the growth of social security-related expenditures within the scope of its natural increase of 610 billion yen (including the pension indexing equivalent) and an increase due to the ageing society of 390 billion yen, system reform and streamlining of 220 billion yen are being implemented. Of the 220 billion yen, what has the most impact is the 160 billion yen worth of “drug price revisions”.
Pharmaceutical Market Only Shrinking in Japan
As a result, while the size of the entire global pharmaceuticals market in fiscal 2020 was approximately 10.3 trillion yen, the growth of the Japanese pharmaceuticals market is rapidly slowing, and in some cases beginning to shrink. In fact, while the growth of the entire market from fiscal 2010 to fiscal 2015 was 3.7%, it was a negative 0.9% growth from fiscal 2015 to fiscal 2020. In addition, what was more serious was that of the approximately 10 trillion yen in drug expenditures (entire pharmaceuticals market), the market for patented products (corresponding to innovative pharmaceuticals) which account for approximately 50% of the market sunk to a negative 0.1% growth from fiscal 2015 to fiscal 2020.
What is the reason behind this situation? It is that while there is autonomous growth which accompanies a basic increase in demand for pharmaceuticals, revisions to drug prices (= drug price reductions) are conducted at a level that exceeds this increase. For example, drug expenditures for fiscal 2019 were approximately 10.6 trillion yen, which was an increase of 0.2 trillion yen by autonomous growth, but this was also impacted by a negative 0.5 trillion yen worth of revision in drug prices, and drug expenditures for fiscal 2020 were approximately 10.3 trillion yen. Moreover, traditional drug price revisions used to be conducted every other year as a rule, but based on “The Basic Policy for Fundamental Reform of the Drug Pricing System” (agreed on Dec. 20, 2014), the revision is now being done every year from fiscal 2021. Thus, according to a forecast by IQVIA Japan, which offers statistical information on the pharmaceutical market, the growth rate for the entire pharmaceutical market for the period fiscal 2020 to fiscal 2026 can only be estimated to be between a negative 0.8% and 0.2%.
IQVIA estimates that while the estimated growth of the pharmaceutical market for the period fiscal 2019 to fiscal 2025 is 4.5% to 7.5% for China and South Korea, 2% to 5% for the United States, 3.5% to 6.5% for Germany, 2.5% to 5.5% for the United Kingdom, 2% to 5% for Italy and Canada, 1.5% to 4.5% for Spain, and 1% to 4% for France, only Japan is forecasted to see largely zero growth or negative growth.
In addition, if Japan, the US, and Europe (average of the UK, Germany and France) were to be compared, of 19 products that were sold between 2016 and 2021, there was only one product which had a high drug price in Japan. Other products are consistently more expensive in the US and in Europe, and the US has traditionally been the top country with the highest number of pharmaceutical items. These basically remain the same, but China surpassed Japan in 2018 and Japan being likely to be surpassed by South Korea is also becoming a serious issue.
In order for innovative drugs to be provided in Japan on a priority and continuous basis ahead of the rest of the world, the Japanese market should remain stable and attractive. This will be of great benefit to the Japanese people, especially patients (Chart 1).
New Drug Innovation Study Group
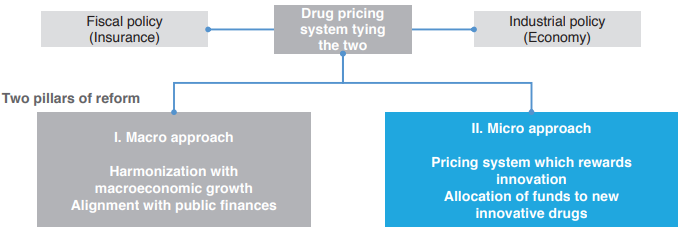
Two Pillars of INES Proposal
With an awareness of such issues, the Institute for New Era Strategy (INES) where I serve on the Board, with the cooperation of experts and companies, established the New Drug Innovation Study Group to examine various issues and propose a framework for a new drug pricing system in order to ensure an environment in which innovative drugs can continue to be provided in Japanese healthcare. The point is that on the premise of maintaining universal health insurance in Japan, in order to balance the burden of medical costs, which are expected to increase in the future with the priority and continuous provision of innovative drugs, it is necessary to introduce a dynamic drug pricing system which balances the appropriate evaluation of the value of innovative drugs with the management of drug costs commensurate with the level of medium- and long-term economic growth. Below provides an overview of the INES proposal.
Core 1 of Micro approach: Drug pricing system reform which rewards innovation
First, the system reform proposal places the drug pricing system as “tying the two” policies of industry policy and fiscal policy (insurance finance), and is composed of two pillars, a micro approach and a macro approach. Of the two, the micro approach aims for a pricing system which rewards innovation and extensive allocation of funds to innovative new drugs. The macro approach aims for harmonization with macroeconomic growth and alignment with public finances.
What specific system reform does the micro approach propose? The INES proposal suggests two reforms in drug price listings and drug price revisions in order to make a drug pricing system which properly evaluates the value of innovative new drugs. First is “introduction of a drug pricing system which properly reflects the value of innovation of new drugs” in drug price listings, and the second is “exemption (abolition) of repricing for market expansion rules” in drug price revisions. The two will be explained in more detail below.
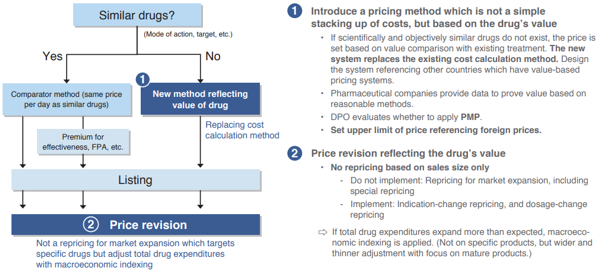
First, “introduction of a drug pricing system which properly reflects the value of innovation”. In order to understand the importance of this method, there is a need to grasp the challenges of the “cost calculation method” upon drug price listings which is currently only adopted in Japan. The cost calculation method is a method that defines drug prices as being the sum of manufacturing costs per drug price setting unit, selling, general and administrative expenses, operating profits, distribution costs, and others. But it has been pointed out that technical difficulties in accurately calculating costs per product are an issue.
For example, the operating profit margin. Any business entity, not just pharmaceutical companies, must raise profits in order to maintain its organization and conduct new investments. Hence, based on a designated standard, the current cost calculation method allows incorporating a fixed rate of operating profit into the cost of the drug price calculation. However, from the standpoint of the pharmaceutical companies, it takes more than a decade for a single product to be launched, and considering the current situation where there are massive failures behind the scenes, there have been deep-rooted claims that drug price calculation based on the cost calculation method unique to Japan does not match the risks associated with the development of drugs (Chart 2).
[Click to enlarge]
To begin with, in transactions based on normal market mechanisms, even when the cost of the product is 100, if consumers determine that it is innovative and valuable, it is not uncommon that the product will be traded at a price more than two or three times the original cost. The reason for Japan introducing its original cost calculation method was to control the impact on health insurance finance, but as stated earlier, in comparing Japan, the US and Europe, of 16 products that were sold after 2016 there is only one which has an expensive drug price in Japan. Under such circumstances, it cannot be said that the Japanese pharmaceutical market is stable or attractive.
Thus, as an introduction of a pricing method which is not a simple stacking up of costs but based on the drug’s value, the INES proposes that if scientifically and objectively similar drugs do not exist, the price be set based on a value comparison with existing treatments. Specifically, it proposes to reference other countries which have value-based pricing systems and foreign prices to set an upper limit on drug prices, while pharmaceutical companies provide data to prove value based on reasonable methods.
Core 2 of Micro approach: Not apply the market expansion repricing rule
The other proposal for system reform is “not apply (abolish) the market expansion repricing rule” upon drug price revision. The market expansion repricing rule lowers the drug price upon drug price revision when annual sales of insurance-listed pharmaceuticals exceed the set multiples of the estimated annual sales. Requirements for activation are (1) more than or equal to twice the estimated annual sales and also when annual sales exceed 15 billion yen, or (2) more than or equal to 10 times the estimated annual sales and also when annual sales exceed 10 billion yen, and the drug prices can be lowered by a maximum of 25%. In addition, for pharmaceuticals whose annual sales are extremely large, there is a “special case for a market expansion repricing rule”, and (1) for annual sales between 100 to 150 billion yen, drug prices can be lowered by a maximum of 25% at 1.5 times or more than the estimated sales, or (2) for annual sales of more than 150 billion yen, drug prices can be lowered by a maximum of 50% at 1.3 times or more than the estimated sales.
In order to promote development of pharmaceuticals which contribute to innovation, whether or not a stable market size for the innovative product can be maintained for a certain period of time after its launch becomes critical. In particular, since in most cases sales hits their peak before the patent expires or in five to six years, which is approximately half of the remaining period, stability of the market size for that period becomes important, but there are many cases where the market expansion repricing rule or a special case rule is activated during the recovery phase of the development costs, and there are many opinions that point to this as being damaging to the drug’s value.
Thus, the INES proposes that market expansion repricing (including special case expansion repricing) based on sales size only be exempt (abolished). Further, this is more trivial but there are rules such as “indication-change repricing” and “dosage-change repricing”. For example, there is a pharmaceutical product called Xolair. The main indication effect of this medication was initially “bronchial asthma”, but it was later found that it was also effective for “seasonal allergic rhinitis”, and when the indication was added the market size quickly expanded, and the special case for indication-change repricing has been activated to raise the drug price.
As such, there does exist a market expansion repricing (including special case expansion repricing) rule that applies to drugs that have undergone changes to their main indications and effects, and this is called “indication-change repricing”. Similarly, a market expansion repricing (including special case expansion repricing) rule for drugs that have seen changes to the dosage is called “dosage-change repricing”.
These repricing rules are greatly impacted by changes to how the drugs are used, and in light of the circumstance that it is slightly different in nature to the autonomous growth of the pharmaceuticals market, the INES proposes that the “indication-change repricing” and “dosage-change repricing” rules be maintained.
Core of Macro Approach: Macroeconomic Indexing of Drug Costs
Of course, if market expansion repricing (including special case expansion repricing) based on sales size were to be excluded (abolished) and if the new drug pricing method based on the value of drugs were to be introduced to replace the “cost calculation method” which stacks up costs, there is a possibility that total drug expenditures will expand beyond estimation. To address this issue, the INES proposal suggests that “macroeconomic indexing of drug costs” be introduced as the other pillar of the system reform – the “macro approach” – and proposes a mechanism that adjusts drug prices broadly and thinly around mature product groups rather than the market expansion repricing that targets specific products.
So what exactly is the “macroeconomic indexing of drug costs” mechanism?
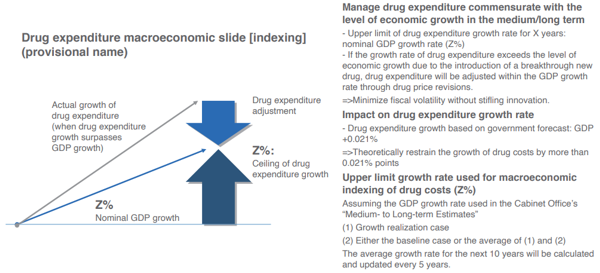
First, the “Medium- to Long-term Estimates” (January 2022) by the Cabinet Office estimate that even in the base line case of low growth, the nominal GDP growth rate will be around 1% until fiscal 2030, and the INES proposes that total drug expenditures grow in line with the medium- to long-term potential economic growth rate.
The main purpose of this is to offset the pessimistic growth rate forecast made by the aforementioned IQVIA Japan for the entire pharmaceutical market for the period fiscal 2020 to fiscal 2026, likely only being a negative 0.8% to negative 0.2 %. This will stabilize the medium- to long-term drug expenditures (against GDP), and elevate the appeal of the Japanese pharmaceutical market (Chart 3).
[Click to enlarge]
However, under the current tight fiscal situation, if total drug expenditures were to grow more than the medium- to long-term potential economic growth rate, it will create various issues. If nominal GDP grew by 1% in the medium- to long-term, tax income and social security income will also grow by 1%. Thus, if total drug expenditures were to grow in line with the medium- to long-term potential GDP growth rate, the issue of additional costs will not arise, but if they grow by more than the potential GDP growth rate, the situation changes. The biggest issue in this case will be the decline in “sustainability of health insurance finance”, but there is a limit to tax increases or raising the social security income, and there is a need to be mindful of the impact on insurers of public health insurance, and the burden on the working generation and the future generations.
How is the government forecasting future trends in drug expenditures (against GDP)? The “Future Prospects for Social Security Toward 2040 (materials for discussion)” (Cabinet Secretariat, Cabinet Office, Ministry of Finance and Ministry of Health, Labour and Welfare, May 21, 2018) which the government published in 2018 may serve as one reference. Medical benefits in fiscal 2018 were approximately 39.2 trillion yen (of which drug expenditures were approximately 10 trillion yen), but according to this forecast medical benefit expenditures (against GDP) which were 7% in fiscal 2018 will expand to 8.9% in fiscal 2040 under the low growth baseline case.
In the approximately 20 years between fiscal 2018 and 2040, medical benefit expenditures (against GDP) are estimated to increase by 1.9 percentage points. Since fiscal 2020, the ratio of drug expenditures to national healthcare costs has seen a certain range (20.2% to 22.6%), but if it is assumed to be around 22%, drug expenditures (against GDP) will expand by 0.42 percentage points (= 1.9 percentage points × 0.22) in the approximately 20 years between fiscal 2018 and 2040. This means that drug expenditures (against GDP) will increase by an annual average of 0.021 percentage points (= 0.42 percentage points ÷ approximately 20 years), and the growth in drug expenditures is “nominal GDP growth rate (Z%) + 0.021%”.
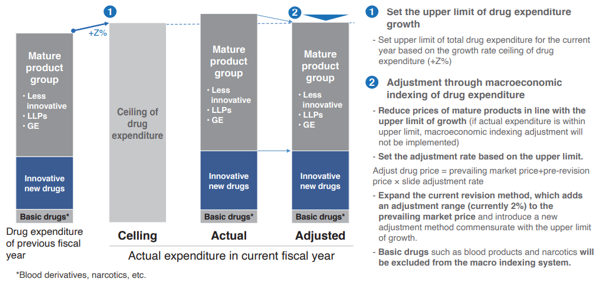
Hence, with an introduction of macroeconomic indexing of drug costs, drug expenditures will grow along with the potential GDP growth rate, and if the growth rate of drug expenditures exceeds the nominal GDP growth rate with the entrance of a revolutionary blockbuster drug, drug prices will be adjusted to fall within the range of the GDP growth rate through drug price revision (Chart 4).
[Click to enlarge]
To be more specific, the following methods will be used to adjust the growth of drug expenditures within the upper limit of the GDP growth rate. First, based on the agreed drug expenditure growth rate (Z%), set a total drug expenditure size for the current fiscal year. Next, drug expenditures will be divided into three groups: “innovative drug group (①)”, “mature product group (②)”, and “basic drugs (③)”. If the growth in drug expenditures exceeds the agreed drug expenditure growth rate (Z%), activate the macroeconomic indexing of drug costs, and by adjusting the drug price for ② (mature product group), adjustments can be made according to the agreed total drug expenditure. In other words, macroeconomic indexing of drug costs is only activated for “mature product groups”, and not for ① or ③.
Explaining this in more detail, aside from macroeconomic indexing of drug costs, annual drug price revisions are conducted for all pharmaceutical products in ①, ②, and ③, and first establish that “adjusted drug price = prevailing market price + pre-revision price × slide adjustment rate”. In addition, when activating the macroeconomic indexing, further trim the slide adjustment rate (currently 2%) for ②, and establish a slide adjustment rate in line with the agreed total drug expenditures.
In other words, in order to restrain drug expenditures within the agreed total drug expenditures, the adjustment rate for the mature product groups at 2% will be trimmed to 1.7%, for example, by slide adjustment. For mature product groups, this means that the current revision method which adds an adjustment range (currently 2%) to the prevailing market price will be expanded, and introduce a new adjustment method commensurate with the upper limit of growth as “adjusted drug price = prevailing market price + pre-revision price × slide adjustment rate”. It should be noted that if total drug expenditure is smaller than the growth rate ceiling, adjustment through macroeconomic indexing of drug costs will not be implemented and total drug expenditure will grow in line with the growth rate ceiling.
Specific Case of Simulation Results
Following the above rules, the INES used the IQVIA data and analyzed the case for fiscal 2020 when macroeconomic indexing of drug costs was activated, and Chart 5 shows the findings.
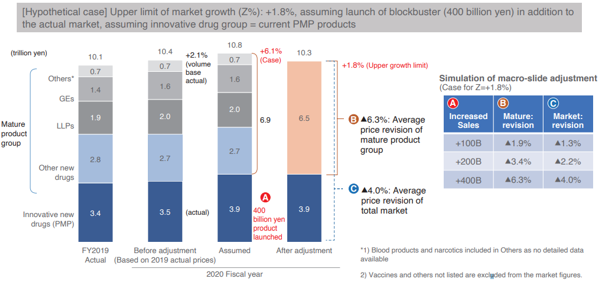
[Click to enlarge]
Specific details will be omitted, but with the additional sales of blockbuster pharmaceutical products such as Opdivo, even if sales of innovative drug groups increased by 400 billion yen, the average drug price revision rate for the entire pharmaceutical market will be a negative 4.0% and the average drug price revision rate for the mature product group will remain at a negative 6.3%, thereby confirming from the chart that even in such extreme cases, the revision rate will be moderate compared to the current drug price revision.
The biggest reason is that while the macroeconomic indexing of drug costs is effective in eliminating financial uncertainties exerted on future health insurance finance, it is effective in increasing total drug expenditures in line with the medium- to long-term potential GDP growth rate.
Above is the overview of the INES proposal on drug pricing system reform, and we do not consider our proposal to be perfect, but rather a “basis” for discussions towards a system reform. As infections of novel coronavirus expand, the importance of the development of vaccines and treatment drugs has once again come to be acknowledged. In order to secure an environment where innovative pharmaceuticals will continue to be offered, further discussions on system reform that enables balancing the promotion of the pharmaceuticals industry and sustainable health insurance finance are anticipated.
This article first appeared on the July/August 2022 issue of Japan SPOTLIGHT![]() published by Japan Economic Foundation. Reproduced with permission.
published by Japan Economic Foundation. Reproduced with permission.
July/August 2022 Japan SPOTLIGHT


